Why was the Congressional Budget Office hacked?
How did Tesla's $1 trillion pay package gain approval?
What impact will FAA's 10% flight cuts have?
Why was Grand Theft Auto VI delayed again?
How will Trump's deal affect weight loss drug prices?
What caused October's highest layoffs in 22 years?
Why is Ford considering ending the F-150 Lightning?
Botox Warning Issued by FDA After Cases of Rare Disease Identified
newsweek.com/botox-warning-fda-cases-rare-disease-botulism-10999636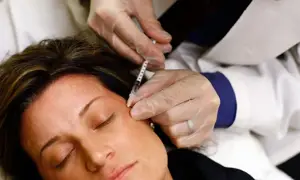
The U.S. Food and Drug Administration (FDA) is warning companies to stop marketing unapproved Botox products after reports of a rare but serious illness linked to botulism.
Why It Matters
Botulinum toxin, commonly called Botox, is used to temporarily reduce wrinkles by paralyzing facial…
This story appeared on newsweek.com, 2025-11-05 21:01:50.